About sleep
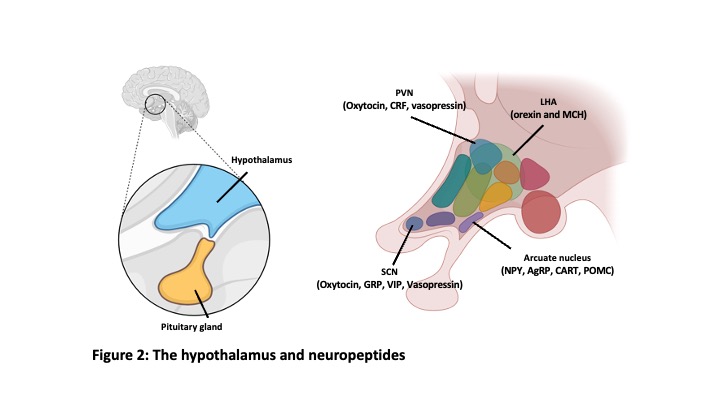
We sleep every day. If we sleep 8 hours a day and assume 80 years of life, we will spend 27 years (30%) of our lifetime sleeping. Sleep initiated from non-rapid eye movement sleep (NREM sleep) and REM sleep follows (Figure 1). These two sleep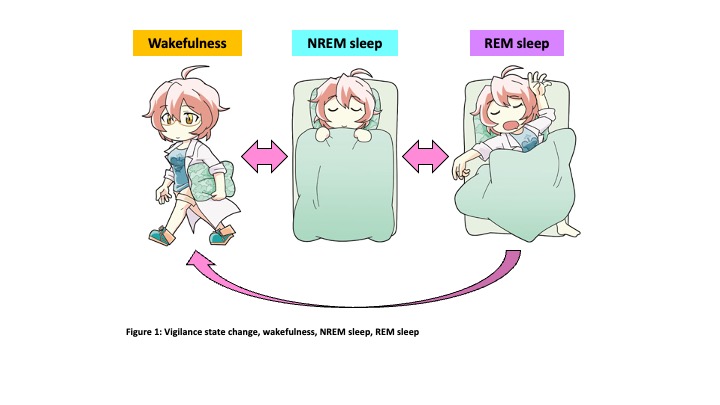 states are completely different in the point of brain activity. However, the regulatory mechanisms, and functions of NREM sleep and REM sleep are still not fully understood. We use mice as experimental animals and state-of-the-art experimental techniques to unravel this mystery. The hypothalamus is known to play a critical role in the regulation of sleep/wakefulness. And recent studies revealed neuropeptide-producing neurons such as orexin neurons and melanin-concentrating hormone (MCH) neurons in the hypothalamus are involved in sleep/wakefulness regulation. (Figure 2)
states are completely different in the point of brain activity. However, the regulatory mechanisms, and functions of NREM sleep and REM sleep are still not fully understood. We use mice as experimental animals and state-of-the-art experimental techniques to unravel this mystery. The hypothalamus is known to play a critical role in the regulation of sleep/wakefulness. And recent studies revealed neuropeptide-producing neurons such as orexin neurons and melanin-concentrating hormone (MCH) neurons in the hypothalamus are involved in sleep/wakefulness regulation. (Figure 2)
Orexin neurons
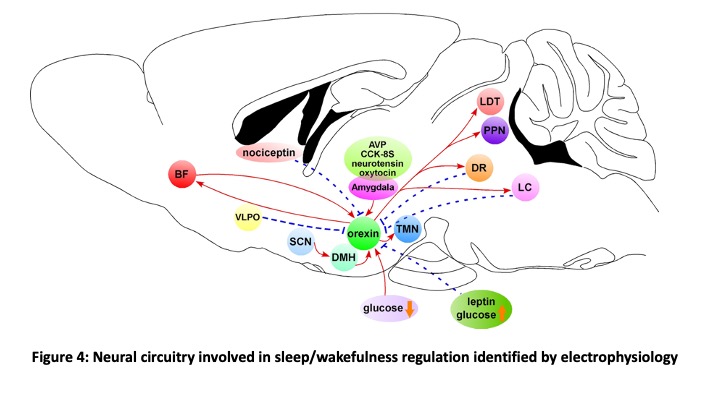
Orexin is a neuropeptide discovered in 1998 (Sakurai et al., Cell 1998). Mature peptides, orexin A and B, are generated from the same precursor protein, prepro-orexin. Orexin A and B act on two types of G-protein coupled receptors, OX1R and OX2R 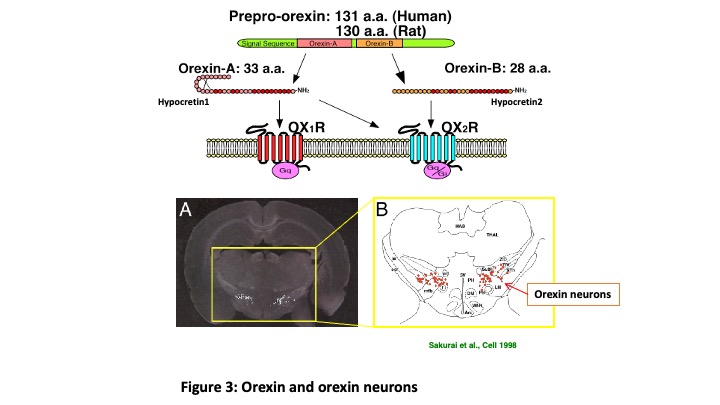 (Figure 3). Orexin is produced in the small number of neurons exclusively located in the hypothalamus. These orexin-producing neurons (orexin neurons) project throughout the brain except for cerebellum. Specific degeneration of orexin neurons is known to be caused by sleep disorder “Narcolepsy” (Peyron et al., Nat Med 2000). Prevalence rate of Narcolepsy is 1/1000-2000 and is known to be more prevalent during puberty. Symptoms of narcolepsy are excessive daytime sleepiness, sleep onset REM sleep, hypnagogic hallucination and cataplexy. By clarifying the input-output pathway of orexin neurons, we can identify the neural circuits involved in sleep/wakefulness regulation. To this end, we generated transgenic mice in which orexin neurons exclusively express green fluorescent protein in the orexin neurons (orexin/EGFP mice) (Yamanaka et al., Neuron 2003). We identify orexin neurons under fluorescent microscopy and recorded activity using slice patch clamp recordings. We applied monoamines (noradrenaline, serotonin and dopamine) and these monoamine neurotransmitter inhibited activity of orexin neurons (Muraki et al., J Neurosci 2004; Yamanaka et al., J Neurophysiol 2006). We also found that orexin activated orexin neurons through the orexin 2 receptor (OX2R) (Yamanaka et al., J Neurosci 2010). These electrophysiological studies revealed neural circuitry which regulates sleep/wakefulness (Figure 4).
(Figure 3). Orexin is produced in the small number of neurons exclusively located in the hypothalamus. These orexin-producing neurons (orexin neurons) project throughout the brain except for cerebellum. Specific degeneration of orexin neurons is known to be caused by sleep disorder “Narcolepsy” (Peyron et al., Nat Med 2000). Prevalence rate of Narcolepsy is 1/1000-2000 and is known to be more prevalent during puberty. Symptoms of narcolepsy are excessive daytime sleepiness, sleep onset REM sleep, hypnagogic hallucination and cataplexy. By clarifying the input-output pathway of orexin neurons, we can identify the neural circuits involved in sleep/wakefulness regulation. To this end, we generated transgenic mice in which orexin neurons exclusively express green fluorescent protein in the orexin neurons (orexin/EGFP mice) (Yamanaka et al., Neuron 2003). We identify orexin neurons under fluorescent microscopy and recorded activity using slice patch clamp recordings. We applied monoamines (noradrenaline, serotonin and dopamine) and these monoamine neurotransmitter inhibited activity of orexin neurons (Muraki et al., J Neurosci 2004; Yamanaka et al., J Neurophysiol 2006). We also found that orexin activated orexin neurons through the orexin 2 receptor (OX2R) (Yamanaka et al., J Neurosci 2010). These electrophysiological studies revealed neural circuitry which regulates sleep/wakefulness (Figure 4).
To mimic narcolepsy in mice, we developed narcolepsy model mice (orexin-tTA; TetO diphtheria toxin A (DTA)) in which orexin neurons are ablated with timing controlled manner in the presence or absence of doxycycline (Tabuchi et al., J Neurosci 2014). These mice reproduced almost all symptoms of narcolepsy. Around 85% ablation of orexin neurons induced fragmentation of sleep/wakefulness and more than 95% ablation induced cataplexy-like behavior arrest suggesting relationship between remaining orexin neurons and appeared symptoms. To further reveal the role of orexin neurons in sleep/wakefulness regulation, we applied optogenetic neural activity manipulation using transgenic mice in which human prepro-orexin promoter driven expression of orange light activated chloride pump, halorhodopsin. Inhibition of orexin neurons in vivo by illuminating orange light into the hypothalamus induced sleep in mice suggested the importance of activity of orexin neurons in the maintenance of wakefulness (Tsunematsu et al., J Neurosci 2011). However, a small number of orexin neurons and sparse distribution make it difficult to record activity in vivo. To record the activity of orexin neurons across sleep/wakefulness state change, fluorescent calcium indicator, GCaMP6, is exclusively expressed in orexin neurons using orexin-Flippase mice (Chowdhury et al., eLife 2019) and adeno-associated virus (AAV) vector. Applying fiberphotometory and microendoscope (nVista), we are recording the activity pattern of orexin neurons and revealing its role in the regulation of sleep/wakefulness (Ito et al., under submission).
MCH neurons
Melanin concentrating hormone (MCH) is 19 amino acid cyclic peptides, originally isolated pituitary of salmon as a hormone which induces concentrating melanocyte to alter color of skin. MCH is well conserved in the mammalian and MCH is expressed in a small number of neurons in the hypothalamus. MCH neurons produce and release MCH as a neurotransmitter together with other neurotransmitters such as glutamate. Similar to orexin neurons, MCH neurons project throughout the brain. Intracerebroventricular injection of synthesized MCH peptide induced feeding behavior suggested involvement in the feeding regulation. However, its physiological function was not clear. To reveal this, we generated transgenic mice in which tetracycline transactivator (tTA) is exclusively express in the MCH neurons (Tsunematsu et al., J Neurosci 2014) and bred them with other transgenic mice which express channelrhodopsin2 (ChR2) in the presence of tTA, KENGE-tet mice (Tanaka et al., Cell Reports 2012), to generate bigenic mice (MCH-tTA; TetO ChR2). Activation of MCH neurons by applying blue light pulse (10 Hz) illumination into the hypothalamus induced REM sleep. These results suggested that MCH neurons are involved in sleep/wakefulness regulation as well, especially in the regulation of REM sleep. Histochemical studies revealed that MCH neurons densely innervate the hippocampus and MCH neurons are dominant neurons projecting hippocampus in the hypothalamus. This result suggested involvement of MCH neurons in the memory regulation as well since the hippocampus is known as the center of memory. We generated MCH neurons ablated mice (MCH-tTA; TetO DTA mice) and evaluated memory using novel object recognition tests. Surprisingly, MCH neurons ablated mice showed improvement of memory ability in the point of acquisition and retention. Optogenetic and chemogenetic manipulation of MCH neurons confirmed that activation and inhibition of MCH neurons impaired and improved memory, respectively. To reveal the activity pattern of MCH neurons, GCaMP6 is expressed in MCH neurons and subjected to fiberphotometory and microendoscopic imagings. And we found that MCH neurons can be classified into three types from its activity pattern, that is wake-active, REM-active and wake-REM-active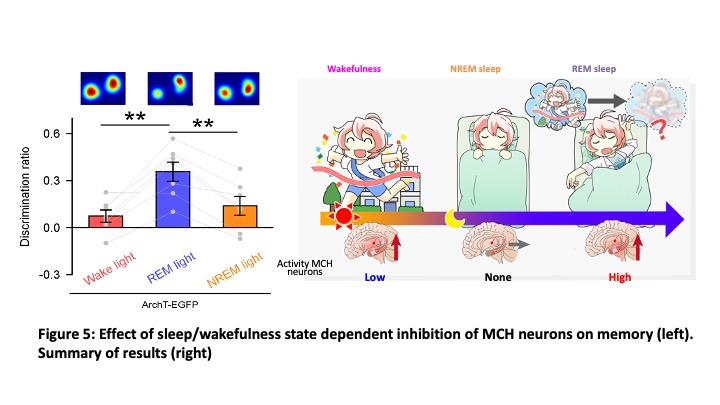 MCH neurons. To test which type of MCH neurons is involved in memory impairment, MCH neurons were sleep/wakefulness state-dependently inhibited by using closed-loop during memory retention for 14 hrs. Although inhibition of MCH neurons during REM sleep improved memory, inhibition during wakefulness or NREM sleep did not improve memory suggesting that MCH neurons active in REM sleep are involved in impair memory (erasure memory or forgetting) (Figure 5, Izawa et al., Science 2019). Brain slice recording from pyramidal neurons in the hippocampus revealed that optogenetic activation of MCH nerve terminals in the hippocampus increased amplitude and frequency of inhibitory post-synaptic currents (IPSCs). This might be one mechanism of memory erasure. Now we are working on identifying the responsible molecule for memory erasure since MCH neurons release not only MCH peptide but also glutamate, cocaine-amphetamine related transcript (CART) etc.
MCH neurons. To test which type of MCH neurons is involved in memory impairment, MCH neurons were sleep/wakefulness state-dependently inhibited by using closed-loop during memory retention for 14 hrs. Although inhibition of MCH neurons during REM sleep improved memory, inhibition during wakefulness or NREM sleep did not improve memory suggesting that MCH neurons active in REM sleep are involved in impair memory (erasure memory or forgetting) (Figure 5, Izawa et al., Science 2019). Brain slice recording from pyramidal neurons in the hippocampus revealed that optogenetic activation of MCH nerve terminals in the hippocampus increased amplitude and frequency of inhibitory post-synaptic currents (IPSCs). This might be one mechanism of memory erasure. Now we are working on identifying the responsible molecule for memory erasure since MCH neurons release not only MCH peptide but also glutamate, cocaine-amphetamine related transcript (CART) etc.
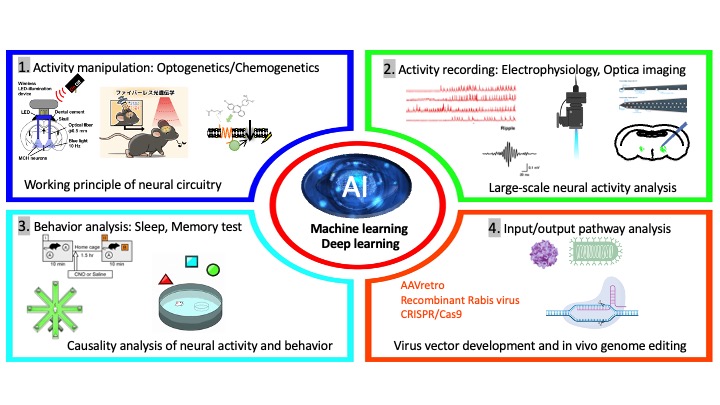
For these studies, we apply neural activity activity manipulation such as optigenetics or chemogenetics and neural activity recordings by using electrophysiology and optical imagings to the targeted neurons. During manipulation or activity recording, we perform behavior experiments such as sleep recordings, learning and memory tests to reveal causality between manipulation and exhibited behaviors. For identification of neural circuitry, trans-synaptically and retrogradely infected virus vectors are used. Connecting these analysis with AI-based analysis, we will reveal the regulatory mechanism of instinctive behaviors (Figure 6).